Energy storage devices like batteries are ubiquitous. From cell phones to hearing aids to automobiles, practically every aspect of modern life is powered by these portable energy sources.
People use batteries all the time, but they are often forgotten. I mean, stop and think about it: are you familiar with how a battery operates? Are you able to elaborate on that for another person?
Here’s a quick primer on the science underlying the fuel that runs everything from smartphones and laptops to electric automobiles and pacemakers. Comparison Test: Gas vs. Electric Cars
A battery is…what?
A battery is a portable chemical power source that stores and releases energy as needed to generate electricity. Unlike conventional electricity, which swiftly transfers energy from a power plant to your home via wires, a battery turns chemicals stored within it into electrical energy, which is then gradually released over the course of days, weeks, months, or even years.
Related How to Unlock Myspace?
There is nothing revolutionary about the concept of portable power; people have always had ways of generating energy while on the go. Even prehistoric people burned wood to create fire, which is another technique of extracting heat energy from chemicals (fire is produced by a chemical reaction known as combustion). By the time of the Industrial Revolution (the 18th and 19th centuries), humans had perfected the process of using coal to generate electricity, allowing steam locomotives and other such machines to run. A locomotive’s boiler normally takes several hours to get hot enough to create steam, and gathering enough wood to cook a meal can take an hour. In contrast, batteries provide us with instant, transportable energy; when you turn the key to your electric automobile, it begins moving in a matter of seconds.
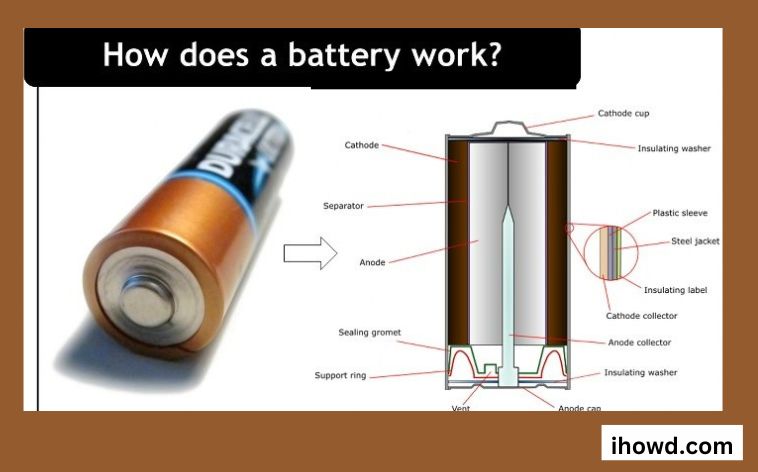
About how batteries function.
Batteries are a common item that most people overlook. They are an unremarkable, daily fixture that quietly stores energy and does its magic behind the scenes. Items such as automobile batteries, cell phone chargers, remote controls, hearing aids, and electric vehicles fall under this category.
However, have you ever taken the time to learn the inner workings of the devices that power our smartphones and other portable electronics instantly? Batteries are portable, self-sufficient power sources that transform chemical energy into electricity. Electrochemistry describes this method. Let’s go into the specifics of what goes into a battery to better explain how it works.
Related How to Unblock Websites?
Can Batteries Also Serve As Capacitors?
There have been many advancements in energy storage in recent years. Batteries and capacitors are currently the most common methods used to store energy. They share a lot of similarities, yet they are not the same. Let’s compare and contrast the two to see how they’re alike and how they’re different.
Three components make up a battery.
One with a positive charge
In electrical terms, a negative electrode
Electrolyte, n.
Each electrode has a separate termination that protrudes from the battery.
Explain the function of each part of a battery?
There are three individual parts to the battery, and the two terminals are plated with a mixture of chemicals and everyday metals. Each terminal has a specific function: anode, cathode, and electrolyte. A chemical layer between a battery’s cathode and anode, the electrolyte, controls and permits the flow of electrical current.
Explain how a battery causes an electric light to shine.
When a light bulb is wired to a battery, a chemical reaction takes place between the electrodes, resulting in a current of electricity that flows to the device. In particular, during an electrical discharge, the chemical at the anode undergoes an oxidation process in which electrons are transferred to the negative terminal and ions within the electrolyte.
Related How to Make a Web Show?
At the same time, electrons are being transferred from the negative terminal to the cathode, creating a circuit that can control and direct the flow of electrons. A primary function of the electrolyte is to provide physical contact between the various chemicals at the anode and cathode. Between one terminal, the chemical potential might remain stable, converting excess chemical energy into usable electrical current.
What is the mechanism of action of rechargeable batteries?
In order to restore the chemical system and replace the battery charge, rechargeable batteries—like those found in most smartphones or automobiles—are designed to receive electric energy from an external power source, often your wall charger.
Can you explain how a battery that cannot be recharged functions?
If the chemical potential of both electrodes in a non-rechargeable or disposable battery remains constant, the battery will continue to supply energy indefinitely. The chemical process that takes place in primary or non-rechargeable batteries is one-way and cannot be reversed.
Conclusion
Now that we’ve answered the most basic question about how batteries work, you can teach everyone else. Batteries and solar panels are covered in more depth elsewhere on the site, should you wish to learn more.